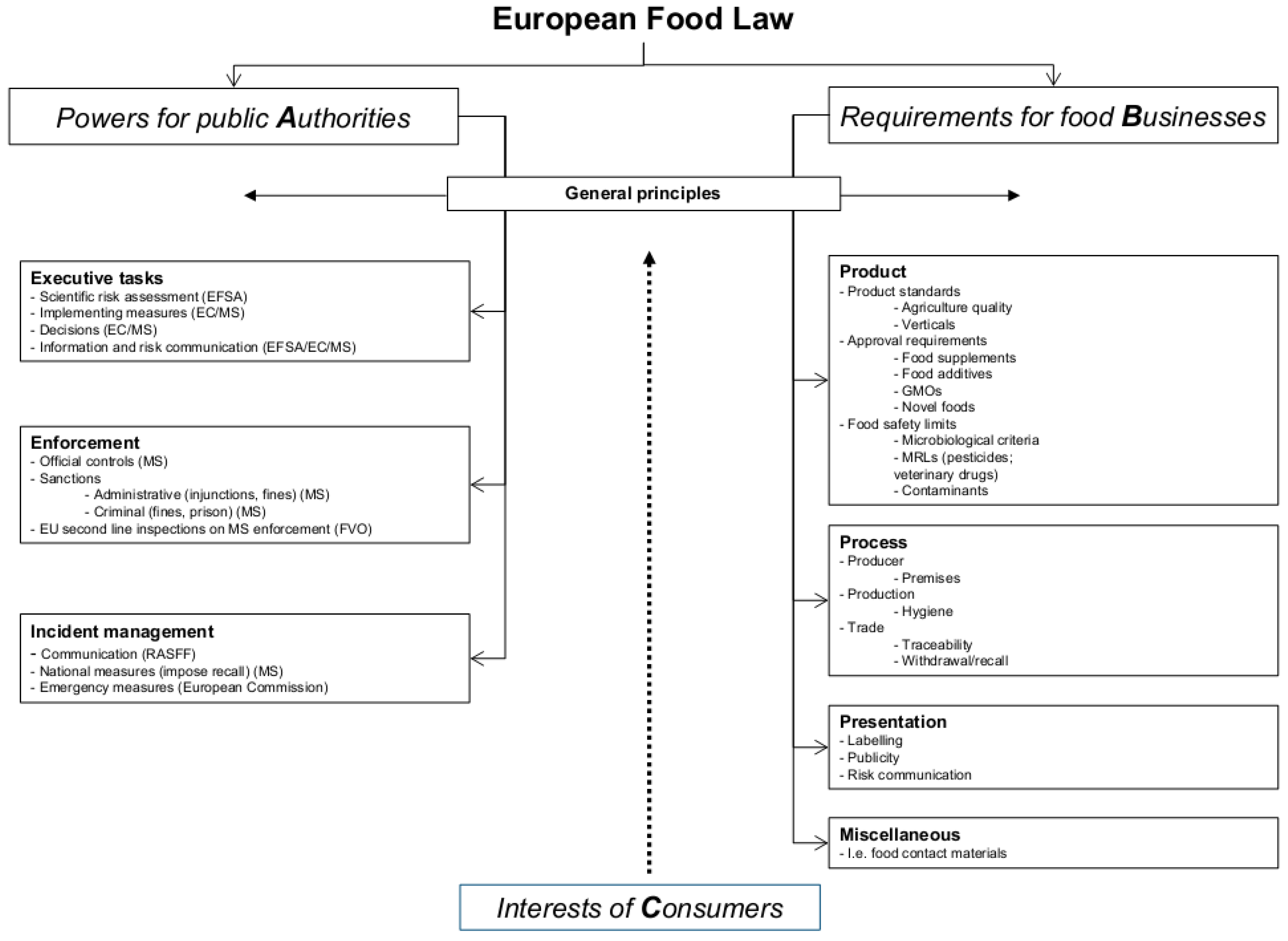
42 information that must be lawfully provided on food labels
Food Labeling: Nutrient Content Claims; Alpha-Linolenic Acid ... To enable the public to comprehend the information provided in nutrient content claims and to understand the relative significance of that information in the context of the daily diet, as required by section 403(r)(2)(G)(iv) of the FD&C Act, qualifying ALA levels for nutrient content claims in food labeling must be based on a single nutrient ... Labeling Requirements - Exemptions From Adequate Directions For Use - Its labeling bears information for use including, indications, effects, routes, methods, and frequency and duration of administration, and any relevant hazards, contraindications, side effects,...
eCFR :: 21 CFR Part 201 -- Labeling The Code of Federal Regulations (CFR) is the official legal print publication containing the codification of the general and permanent rules published in the Federal Register by the departments and agencies of the Federal Government. The Electronic Code of Federal Regulations (eCFR) is a continuously updated online version of the CFR. It is not an official legal edition of the CFR.

Information that must be lawfully provided on food labels
21 CFR Part 101 -- Food Labeling - eCFR The principal display panel shall be large enough to accommodate all the mandatory label information required to be placed thereon by this part with clarity ... Nutrition Labelling - Centre for Food Safety 1 Feb 2021 — Nutrition label must include the information on energy and seven nutrients specified for labelling (1+7), namely, protein, carbohydrates, ... eCFR :: 21 CFR Part 812 -- Investigational Device Exemptions When the sponsor receives from the IRB information concerning the public disclosures under § 50.24(a)(7)(ii) and (a)(7)(iii) of this chapter, the sponsor shall promptly submit to the IDE file and to Docket Number 95S-0158 in the Division of Dockets Management (HFA-305), Food and Drug Administration, 5630 Fishers Lane, rm. 1061, Rockville, MD 20852, copies of the information …
Information that must be lawfully provided on food labels. eCFR :: 21 CFR Part 801 -- Labeling 801.20 - 801.57. § 801.20. Label to bear a unique device identifier. § 801.30. General exceptions from the requirement for the label of a device to bear a unique device identifier. § 801.35. Voluntary labeling of a device with a unique device identifier. § 801.40. Form of a unique device identifier. FOOD LABELING - GovInfo a food, including foods that comply with standards of identity, except those ingredients exempted by § 101.100, shall be listed by common or usual name in ... FDA Regulation of Cannabis and Cannabis-Derived Products ... In addition, under 21 CFR 530.20(b)(2), if scientific information on the human food safety aspect of the use of the approved human drug in food-producing animals is not available, the veterinarian ... The Food Labelling Regulations 1996 - Legislation.gov.uk (1) If the claim is that the food is a rich or excellent source of protein, at least 20 per cent of the energy value of the food must be provided by protein. (2) In any other case, at least 12 per cent of the energy value of the food must be provided by protein. 3. The food must be marked or labelled with the prescribed nutrition labelling.
Food Label News Reader Questions - FAQ for FDA Regulations on Food Labels All ingredients that are used in a food must be disclosed in the Ingredient Statement on the label. Ingredients must be listed in descending order of predominance by weight. A statement such as "Contains 2% or less:" may be used at the end of the Ingredient Statement and ingredients thereafter do not need to be listed in order of predominance. TECH207_Week 1_Tutorial_Food and energy_Food labels.pdf Food is comprised of a variety of nutrients that are essential for good health and normal growth and development. Nutrients can be divided into 2 major groups, macronutrients and micronutrients. • Macronutrients are required in large amounts (usually gram amounts). The essential macronutrients are water, carbohydrates, proteins and fats (lipids). World-Class Regulatory Consulting Services- I3CGLOBAL FDA Label review (Food, Drugs, Cosmetics) means verification of existing labels by our experts against FDA’s labeling regulation READ MORE FDA Agents are the primary contact point person or company between the foreign facility and the FDA in case of any emergency and routine registration matters. CFR - Code of Federal Regulations Title 21 - Food and Drug … Mar 29, 2022 · A reference to information submitted previously must identify the file by name, reference number, volume, and page number where the information can be found. A reference to information submitted to the agency by a person other than the sponsor is required to contain a written statement that authorizes the reference and that is signed by the ...
What Information must be lawfully provided on food labels ... - Answers Generally, food labels will contain the nutritional information (calories, fat, carbs, sugars, and so on), along with any vitamins or minerals in the food, the serving size, and the ingredietns in... Exam 1 -Emily (Nutrition) Flashcards Information that must be lawfully provided on food labels includes all of the following except Select one: A. the amount recommended for ingestion each day. B. the amounts of specified nutrients and food components. C. the net contents expressed by weight, measure, or count. D. the name and address of the manufacturer, packer, or distributor NYS Pharmacy:Laws, Rules & Regulations:Part 63 - New York … 20.8.2009 · §63.3 Licensing examinations. Effective January 1, 2022, each candidate applying for licensure as a pharmacist in New York State shall pass an examination or examinations acceptable to the board of pharmacy for licensure purposes and determined by the department to be satisfactory for measuring the applicant's knowledge regarding the curricular areas defined … Membership Information and Guidelines | The Bay Club Cell phone use is permitted only in the Main Lobby, Food & Beverage, and outdoor areas. Please refrain from conversing on speaker phone or FaceTime in public gathering spaces. Silent cell phone use (no phone calls) only is permitted in the Fitness Center and Clubhouses. Ringers must be off on the golf course, driving range, tennis and squash ...
IHCPFAQS - California For example, food product labels must comply with Title 21, CFR Part 101 – Food Labeling. Required information on food labels include a statement of identity, ingredient list in descending order of predominance by weight, net quantity of product in the package, an address for the responsible party and a nutrition facts panel, when applicable.
Nutrition Final Exam Flashcards | Quizlet Information that must be lawfully provided on food labels includes all of the following except... The amount recommended for ingestion each day. What is a bolus? A portion of food swallowed at one time What are two major nutrients supplied by the fruit and vegetable group? Vitamins A and C
Consumer Health Information for Better Nutrition Initiative ... ADA recommends pre-market research of consumer perceptions of the various label layouts, designs and effectiveness of communication strategies be conducted prior to the qualified claim's approval....
Nutrition final Flashcards | Quizlet what is the recommended diet percentage for proteins 20-30% which of the following are examples of carbohydrate-rich foods wheat and lentils because they accumulate in the body, excess ingestion of which of the following can have toxic effects fat-soluble vitamins a hormone produced by the hypothalamus that results in hunger ghrelin
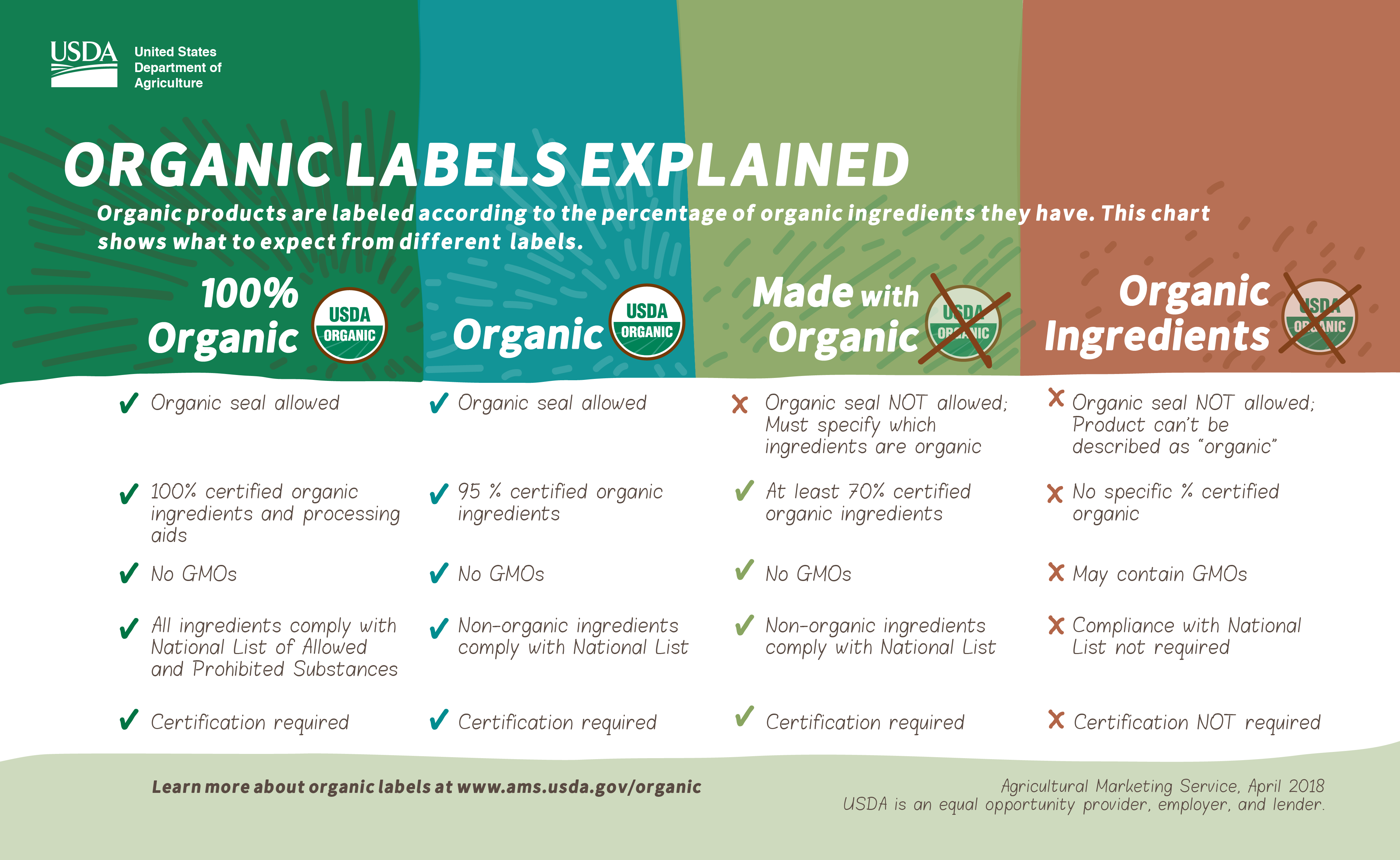
Nutrition labelling - Language selection | Food Safety 20 May 2020 — Which nutrition information is mandatory on food labels? ... The declaration must be presented in a legible tabular format on the packaging.
Which of the following is a characteristic of - Course Hero Information that must be lawfully provided on food labels includes all of the following except: a) the amount recommended for ingestion each day. b) the amounts of specified nutrients and food components. c) the net contents expressed by weight, measure, or count. d) the name and address of the manufacturer, packer, or distributor.
Food safety for food delivery | Food Standards Agency You must provide allergen information: before the purchase of the food is completed - this can be in writing (on a website, catalogue or menu) or orally (by phone) when the food is delivered - this...
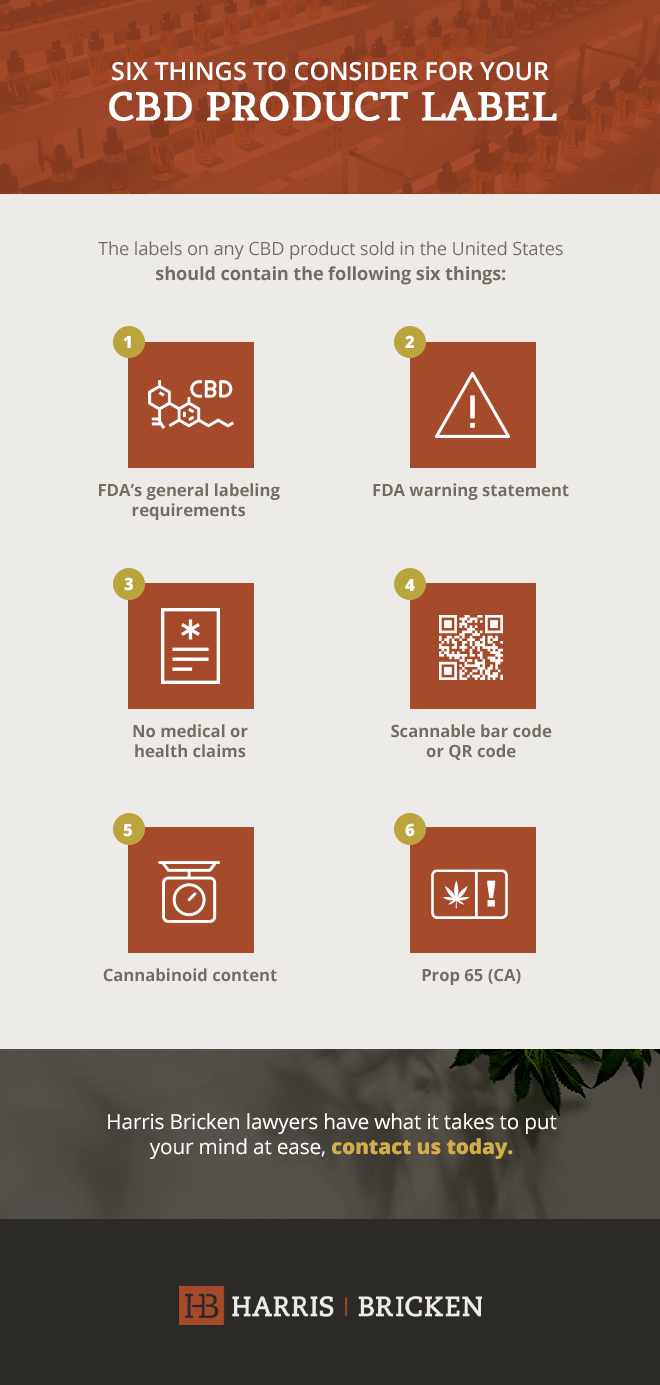
Marijuana Packaging & Labeling Laws by State | Leafly Pre-Approval of Labels (1) A registrant must submit labels for pre-approval in accordance with OAR 845-025-7060 and must keep all records related to the pre-approval process and provide those ...
Nutrition Quiz 2 - Multiple Choice 1. Refined grain... Information that must be lawfully provided on food labels includes all of the following except a. the amount recommended for ingestion each day.b. the amounts of specified nutrients and food components. c. the net contents expressed by weight, measure, or count. d. the name and address of the manufacturer, packer, or distributor End of preview.
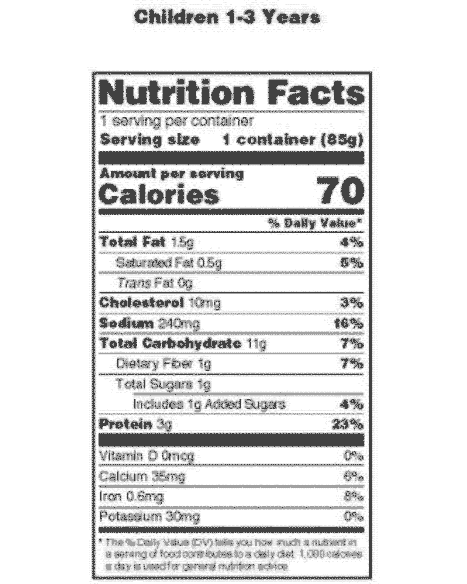
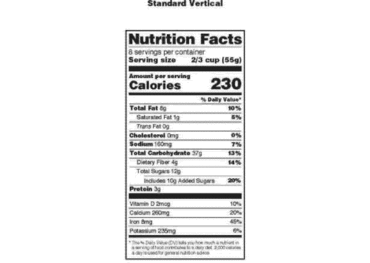
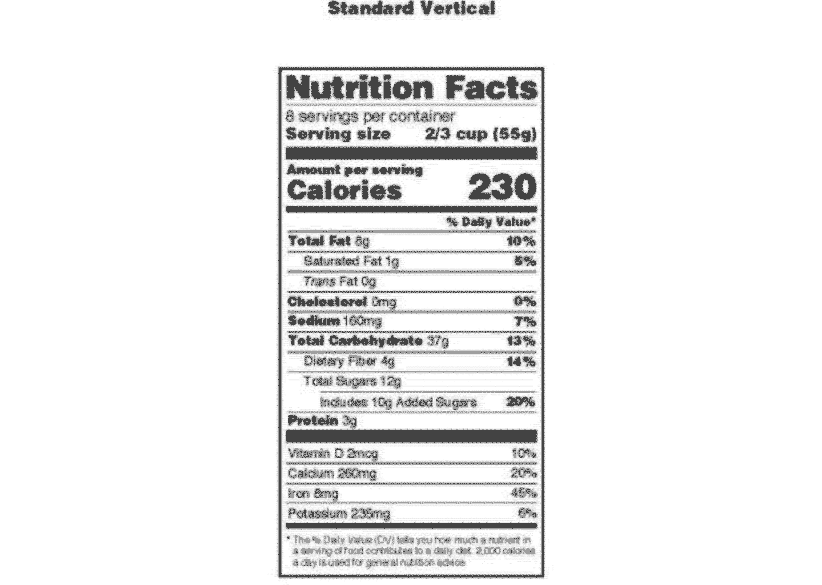
Food Labeling: Revision of the Nutrition and Supplement Facts Labels Following the passage of the Nutrition Labeling and Education Act (NLEA) of 1990 (the 1990 amendments) (Pub. L. 101-535), which added section 403(q) of the Federal Food, Drug, and Cosmetic Act (the FD&C Act) (21 U.S.C. 343(q)), we issued various regulations related to nutrition information on food labels, including the declaration of nutrients ...
Nutrition declaration - EU labelling rules - Your Europe 18 May 2022 — The energy value and nutrient amounts on the package must reflect the value and amounts of the food as it is sold. If the product requires ...
Application for Permanent Residence – Business Immigration Program ... Information on medical instructions will be provided to you by the IRCC office. When you receive your assessment notice you will also receive medical forms for yourself (and any dependants, if applicable ) and instructions on how to access a list of doctors in your area who are authorized to conduct immigration medical examinations (see below).
Guidance for Industry: Food Labeling Guide | FDA Questions concerning the labeling of food products may be directed to the Food Labeling and Standards Staff (HFS-820), Office of Nutrition, Labeling, and Dietary Supplements, Center for Food ...
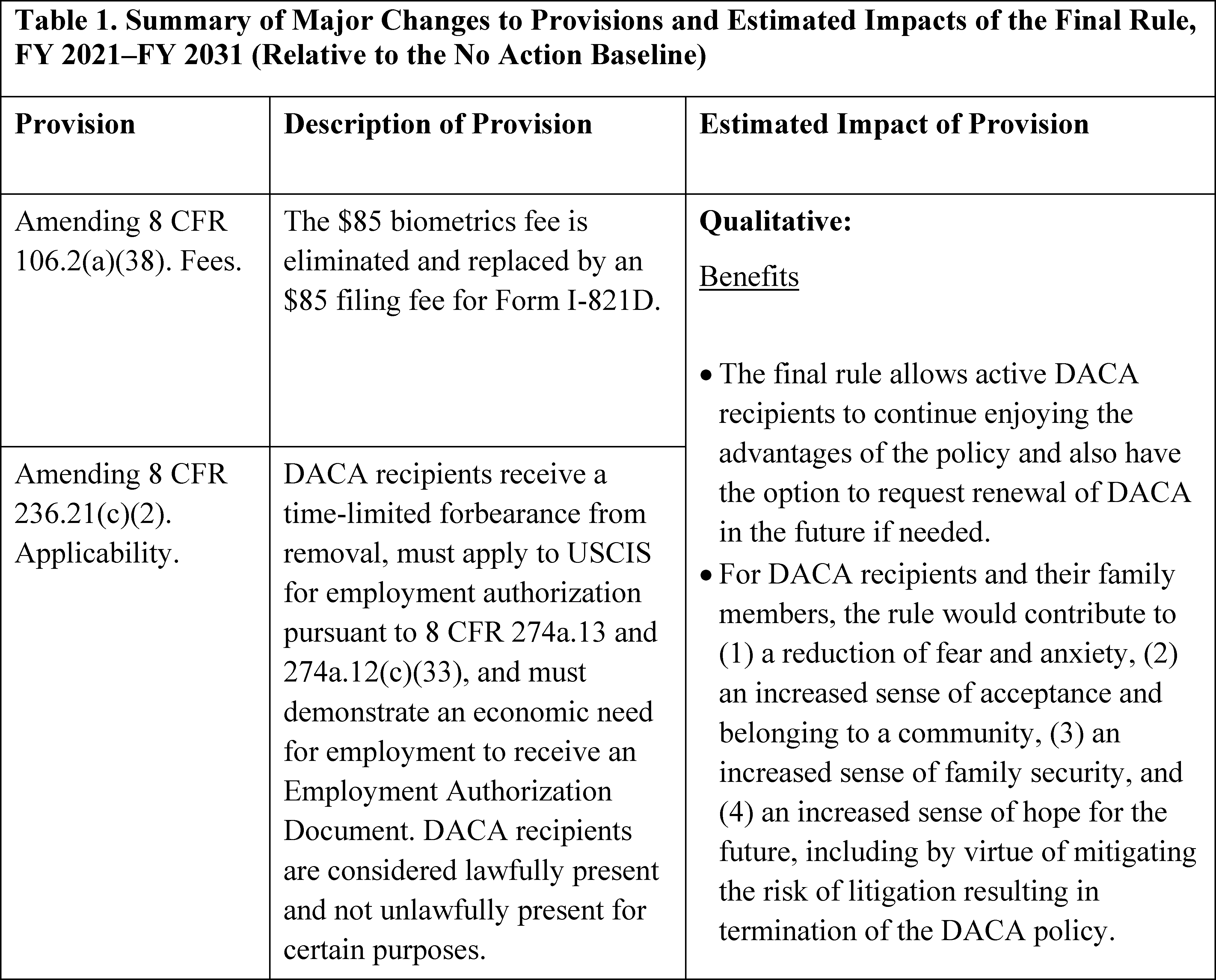
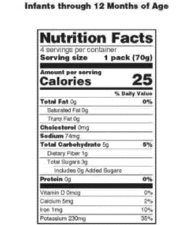
All about Food Labels - HealthCheckSystems The nutritional information given on the label is based on one serving of the food. Nutrition Facts - each package must identify the quantities of specified nutrients and food constituents for one serving. From this information, you can gleam some very useful information. The most important thing to remember is this: 1 gr. fat = 9 calories
Nutrition Ch. 2 **** Flashcards | Quizlet Nutrient dense refers to foods that a. carry the USDA nutrition labeling. b. are higher in weight relative to volume. c. provide more nutrients relative to kcalories. d. contain a mixture of carbohydrate, fat, and protein. c The concept of nutrient density is most helpful in achieving what principle of diet planning? a. Variety b. Balance
Enforcement – Welcome to the City of Fort Worth Call 817-392-1234 and provide the following information: address and description of animal; time of incident; other pertinent details; You will be advised to maintain a complaint log for 7 to 10 days beginning with the date that the complaint is filed. The information in the log must contain the date, times and duration of the noise nuisance.
PRN 2000-5: Guidance for Mandatory and Advisory Labeling Statements Advisory statements generally provide information, either in support of the mandatory statements or about the product in general. To ensure that the intent of each labeling statement is clear, mandatory statements need to be clearly distinguishable from advisory statements.
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration (a) Applicability. Except as provided in this section, this part applies to all clinical investigations of products that are subject to section 505 of the Federal Food, Drug, and Cosmetic Act or to the licensing provisions of the Public Health Service Act (58 Stat. 632, as amended (42 U.S.C. 201 et seq.)). (b) Exemptions. (1) The clinical investigation of a drug product that is lawfully ...
PDF Food Labeling Guide - Food and Drug Administration Food Labeling Guide Additionalcopies are available from: Office of Nutrition, Labeling, and Dietary Supplements HFS-800 Center for Food Safety and Applied Nutrition Food and Drug Administration...
TTBGov - Labeling LABELING, ADVERTISING OR CONTAINERS OF BEVERAGE ALCOHOL PRODUCTS (spirits, wine or beer) - Report this information to the Alcohol and Tobacco Tax and Trade Bureau by e-mail, telephone or writing to: Alcohol and Tobacco Tax and Trade Bureau Alcohol Labeling and Formulation Division 1310 G Street, NW., Box 12 Washington, DC 20005
Part 552 - Solicitation Provisions and Contract Clauses (a) General.When a GSAR provision or clause is used without deviation in a solicitation or contract, it shall be identified by number, title, and date ( e.g., 552.211-77, Packing List (FEB 1996)). (b) Deviations. (1) Federal Acquisition Regulation deviations.When a GSAR provision or clause is used with an authorized deviation in lieu of a FAR provision or clause in a solicitation …
PDF Pennsylvania Department of AGRICULTURE If a label has been approved by PDA, the colors and graphics may be changed without requiring re-approval of the label, provided such changes otherwise comply with this document. If the text, type size or wording is to be changed, the label must be submitted to PDA for approval. 7. Label Representations. (A) No labeling may be false or misleading.
Sales, Advertising and Labelling: General Food Labelling 'descriptive name' means a name providing a description of the food which is sufficiently clear to enable consumers to know its true nature and distinguish it from other products with which it might be confused. 36 it should involve a clear and accurate description of the food using specific not generic terms and which avoids meaningless …
21 CFR § 251.13 - LII / Legal Information Institute The NDC(s) must be included in the HOW SUPPLIED section in place of the NDC(s) assigned to the FDA-approved versions of the drug. The NDC(s) also must be included on the immediate container label and outside package. (c) The Importer is responsible for relabeling the drug, or arranging for it to be relabeled, to meet the requirements of this ...
Nutrition Chapter 2 Flashcards | Quizlet Information that must be lawfully provided on food labels includes all of the following except a. the amount recommended for ingestion each day b. the amounts of specified nutrients and food components c. the net contents expressed by weight, measure or count d. the size of the serving and the number of servings per package
Engenia Herbicide As an over-the-top dicamba with the lowest use rate, Engenia ® herbicide helps you cover more acres more efficiently , so you can focus on tackling more items on your to-do list this season. Powered by BASF's proprietary BAPMA salt and high-performance dicamba, you can be confident that you're getting the most advanced herbicide available for dicamba-tolerant soybeans.
Food exchange systems were originally developed for - Course Hero Information that must be lawfully provided on food labels includes all of the following except a. the amount recommended for ingestion each day. b. the amounts of specified nutrients and food components.c. the net contents expressed by weight, measure, or count. d. the name and address of the manufacturer, packer, or distributor. _ 63.
eCFR :: 21 CFR Part 812 -- Investigational Device Exemptions When the sponsor receives from the IRB information concerning the public disclosures under § 50.24(a)(7)(ii) and (a)(7)(iii) of this chapter, the sponsor shall promptly submit to the IDE file and to Docket Number 95S-0158 in the Division of Dockets Management (HFA-305), Food and Drug Administration, 5630 Fishers Lane, rm. 1061, Rockville, MD 20852, copies of the information …
Nutrition Labelling - Centre for Food Safety 1 Feb 2021 — Nutrition label must include the information on energy and seven nutrients specified for labelling (1+7), namely, protein, carbohydrates, ...
21 CFR Part 101 -- Food Labeling - eCFR The principal display panel shall be large enough to accommodate all the mandatory label information required to be placed thereon by this part with clarity ...
















Post a Comment for "42 information that must be lawfully provided on food labels"